Sickle cell anemia. Cystic fibrosis. These are conditions that you may have heard of in a biology class, on the news, or maybe through a friend. Two genetic conditions you probably have not heard of are Leber’s Hereditary Optic Neuropathy (LHON) and Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes (MELAS). People with LHON experience severe vision loss, tremors, and heart arrhythmias. MELAS causes people to have stroke-like episodes at a young age, seizures, and recurrent headaches and vomiting.
On the surface, these two conditions sound very different. However, both LHON and MELAS have the same underlying cause. As with other genetic conditions, mutations (changes) in our DNA cause people to develop LHON and MELAS. What makes them special is that these conditions are caused by mutations in the mitochondrial DNA.
You may ask, “What’s a mitochondria?” Mitochondria are the batteries of our cells. Each cell has a number of mitochondria that makes the energy it needs to survive. While most of our DNA resides in a part of the cell called the nucleus (special section in the very center of the cell), mitochondria have their own set of DNA that allows them, quite amazingly, to grow, develop, function, and even reproduce on their own.
Since mitochondria play such an important role in our bodies, mutations that impact their ability to make energy have a big effect on our health. So called mitochondrial disorders, which affect as many as 1 in 5,000 people, have the greatest impact on muscle and heart tissue as well as the brain and the rest of the nervous system. That’s because these parts of the body need the most energy. Many people with these conditions have significantly shortened lifespans.
Mitochondrial disorders are inherited a little differently than most other genetic conditions. A dad with a mitochondrial disorder will never pass it to his children. Sperm don’t pass along their mitochondria to the egg, so it only matters if mom has these changes in her mitochondria. As there are many mitochondria in each cell, the amount of mitochondria with a mutation affects how severe a disease may be. The number of affected mitochondria may also change over time, making it difficult to predict the future severity outlook of a disease.
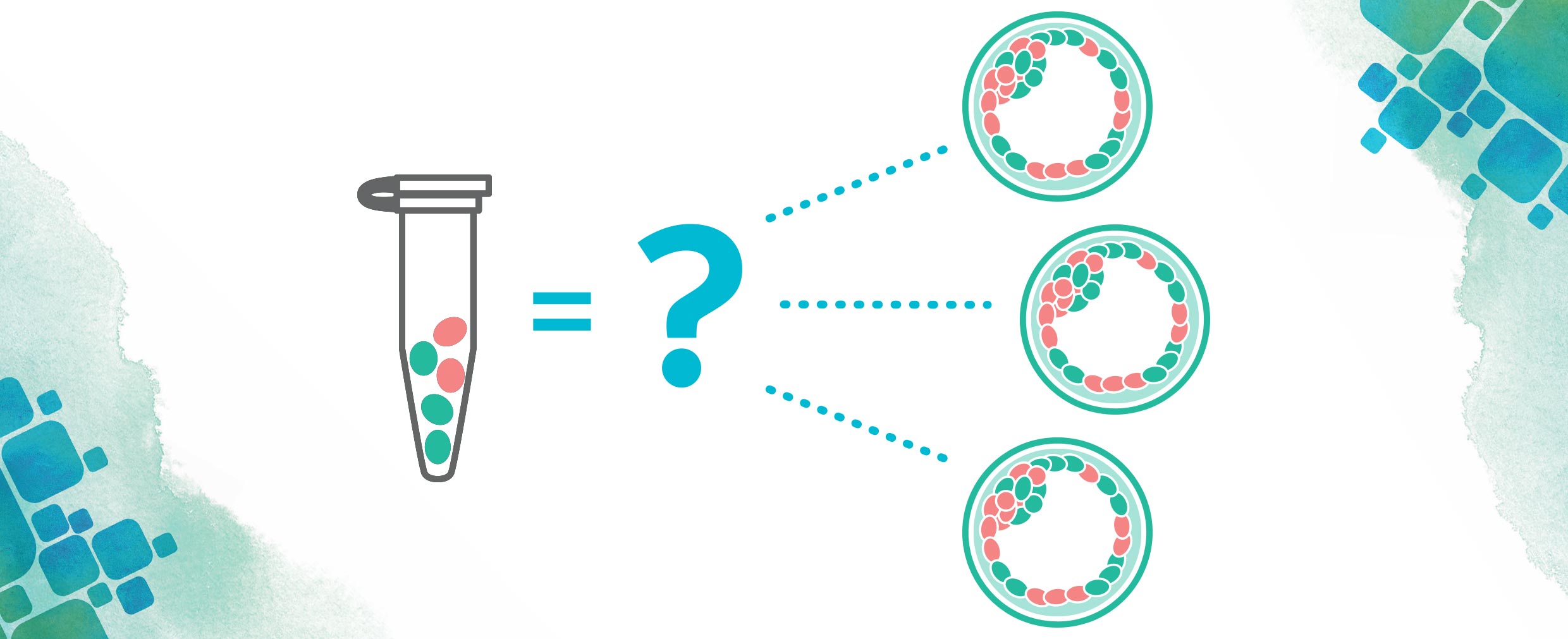
As with any medical condition, there has been a lot of research done into how to cure mitochondrial disorders. One proposed cure involves removing the affected mitochondria in an egg before or shortly after fertilization and replacing these mitochondria with healthy ones. This procedure, called mitochondrial replacement therapy (MRT), would be able to prevent these diseases before they could ever start and is a process done through in vitro fertilization (IVF).
There has been a good amount of controversy regarding MRT in the past few years. Many are worried that tampering with our mitochondrial DNA can lead us towards “designer babies” (think Gattaca). Some are concerned about the idea of “three parent babies” and how this would redefine parenthood. In reality, mitochondrial DNA makes up only about 0.1% of our total DNA and is mostly involved in making all the energy we need to live. Things like eye color or hair color are well outside the realm of the mitochondria!
The safety and usefulness of MRT has also been called into question. Though some research has been done into these issues, many feel MRT has not been properly qualified for medical purposes. As debates about allowing MRT have raged in the U.S. over the past years, the UK took the bold step of granting the first license for MRT in March 2017.
Ultimately, time will tell if this technique provides the benefits that many expect it to have. For now, MRT has helped bring to light many conditions that affect hundreds of thousands of people worldwide. If you would like to learn more about mitochondrial disorders, check out the United Mitochondrial Disorders Foundation (other supporting organisations available, dependant on country).
Rob is a second year genetic counseling student in the Joan H. Marks Graduate Program in Human Genetics at Sarah Lawrence College. While at Sarah Lawrence College, he has served as a student coordinator for the Impact program, which matches genetic counseling students with families having a child with Down syndrome to facilitate a broader understanding of this condition. His interests include public health genetics, education, and raising awareness about genetic conditions. Rob received his B.S. in Molecular Genetics with minors in Psychology and Ethics from the University of Rochester.
Article first published May 2017.